How Do You Think The Size Of Atoms Will Change From Top To Bottom Within A Chemical Family?
In the Periodic Table, there are a number of physical properties that are not actually "like" as it was previously divers, but are more tendency-like. This means is that as you lot movement down a group or beyond a period, you lot volition run across a trend-like variation in the properties. In that location are three specific periodic trends that we volition talk over. The first lesson of this chapter is devoted to the trend in atomic size in the Periodic Tabular array. The two following this lesson will discuss ionization energy and electron affinity. Each of these trends can be understood in terms of the electron configuration of the atoms.
The actual trends that are observed with atomic size have to do with three factors. These factors are:
- The number of protons in the nucleus (called the nuclear charge).
- The number of free energy levels belongings electrons (and the number of electrons in the outer free energy level).
- The number of electrons held between the nucleus and its outermost electrons (called the shielding upshot).
Lesson Objectives [edit | edit source]
- Define atomic radius.
- Land the boundary issue with diminutive size.
- Describe measurement methods for atomic size.
- Define the shielding upshot.
- Describe the factors that determine the tendency of atomic size.
- Describe the general tendency in atomic size for groups and for periods.
- Describe the trend of atomic radii in the rows in the Periodic Table.
- Depict how the trend of atomic radii works for transition metals.
- Use the general trends to predict the relative sizes of atoms.
- Use the concept of constructive nuclear charge to explain why the diminutive radii of the main grouping elements increase when we move down a group in the periodic table
Atoms Accept No Definite Boundary [edit | edit source]
The region in space occupied by the electron cloud of an atom is oft thought of equally a probability distribution of the electrons and therefore, in that location is no well-defined "outer edge" of the electron deject. Atomic size is defined in several different ways and these different definitions ofttimes produce some variations in the measurement of atomic sizes.
Because it is and then difficult to measure atomic size from the nucleus to the outermost edge of the electron deject, chemists utilise other approaches to get consistent measurements of atomic sizes. Ane way that chemists define atomic size is past using the atomic radius. The atomic radius is half the altitude between the centers of a homonuclear diatomic molecule (a diatomic molecule means a molecule made of exactly two atoms and homonuclear means both atoms are the same element). The figure below represents a visualization of the diminutive size definition.

A visual representation of the atomic radius of a hydrogen atom. The measurement would be taken equally half the distance between the nuclei of the hydrogen atoms in a diatomic hydrogen molecule.
How practise we measure the size of the atom? Ernest Rutherford is famous for his experiments bombarding golden foil with alpha particles. The golden foil experiment by Rutherford, kickoff done in 1911, is of particular interest to usa in this unit because information technology was this experiment that first gave science an guess measurement for the size of the atom. He was able, using technology available in the early part of the 1900s, to make up one's mind quantitatively that the nucleus had an guess size of 4×10−12 cm. The size of the atom is slightly larger, approximately ii×10−viii cm in diameter.
Atomic Size in a Column Increases from Summit to Bottom [edit | edit source]
Let's now look at the atomic radii or the size of the atom from the pinnacle of a family or group to the lesser. Take, for example, the Group 1 metals. Each atom in this family (and all other main grouping families) has the aforementioned number of electrons in the outer energy level as all the other atoms of that family. Each row (menses) in the periodic table represents another added energy level. When nosotros get-go learned about primary free energy levels, we learned that each new energy level was larger than the one before. Free energy level ii is larger than energy level 1, energy level iii is larger than free energy level two, and so on. Therefore, as we move down the Periodic Table from period to menstruum, each successive flow represents the add-on of a larger free energy level. Information technology becomes credible that every bit we move downwards through a family of elements, that each new atom has added another energy level and volition, therefore, be larger.

One other contributing cistron to atomic size is something called the shielding effect. The protons in the nucleus attract the valence electrons in the outer free energy level because of opposite electrostatic charges. The strength of this attraction depends on the size of the charges, the distance between the charges, and the number of electrons in-between the nucleus and the valence electrons. The core electrons shield the valence electrons from the nucleus. The presence of the core electrons weakens the allure between the nucleus and the valence electrons. This weakening of the attraction is called the shielding effect. The amount of shielding depends on the number of electrons between the nucleus and the valence electrons. The force with which the nucleus pulls on the valence electrons can pull the valence shell in tighter when the allure is strong and not so tight when the attraction is weakened. The more shielding that occurs, the further the valence vanquish can spread out.
| Element | # of protons | Electron Configuration | # of energy levels |
|---|---|---|---|
| Li | three | [Ne]2south one | 2 |
| Na | xi | [He]3s ane | 3 |
| Chiliad | 19 | [Ar]4south 1 | four |
| Rb | 37 | [Kr] 5s i | 5 |
| Cs | 55 | [Xe]6southward 1 | 6 |
For example, if you lot are looking at the element sodium, it has the electron configuration:
- Na: 1s iitwosouth 22p 6iiis 1
The outer free energy level is due north = 3 and there is one valence electron but the attraction between this lone valence electron and the nucleus that has those xi protons is shielded by the other 10 inner (or core) electrons.
When we compare an atom of sodium to 1 of cesium, we observe that the number of protons increases as well as the number of free energy levels occupied by electrons. There are likewise many more electrons betwixt the outer electron and the nucleus, thereby shielding the allure of the nucleus.
- Cs: 1southward 22due south 22p 6threes two3p 64s iiiiid 10ivp 65s 24d 105p half dozensixsouthward 1
The outermost electron, 6s ane, therefore, is held very loosely. In other words, because of shielding, the nucleus has less command over this 6s ane electron than it does over a 3s i electron. The result of all of this is that the cantlet's size will be larger. Table x.2 gives the values for the atomic radii for the Group 1 metals plus a visual representation to capeesh the size change in a grouping in the periodic table (measurement units for diminutive radii are picometers [pm] or one×10−12 meters).
| Element | Atomic Radii | Visual |
|---|---|---|
| Li | 123 pm |  |
| Na | 157 pm |  |
| K | 203 pm |  |
| Rb | 216 pm |  |
| Cs | 235 pm |  |
What is true for the Grouping ane metals is true for all of the groups, or families, across the periodic table. Equally y'all move downward in the periodic table through a family unit group, the size of the atoms increases. For instance, the atoms that are the largest in the halogen family are bromine and iodine (since astatine is radioactive and just exists for brusque periods of time, we won't include information technology in the word).
Sample Question
Which of the following is larger? Explicate.
- (a) As or Sb
- (b) Ca or Be
- (c) Po or S
Solution:
- (a) Sb because it is beneath As in Group 15.
- (b) Ca because it is below Be in Group two.
- (c) Po because it is below S in Grouping xvi.
As noted earlier for the main group metals, the outermost free energy level in the electron configuration is indicated past the period number. And so the chemical element magnesium (Z = 12), is in Menstruation three, Group 2. Co-ordinate to this, we can say that there are 3 free energy levels with two electrons in the outermost energy level. Allow'due south look at the electron configuration for magnesium.
- Mg: is twotwos ii2p 63s 2
Moving from magnesium to strontium, strontium is in the 5th menstruum of Group two. This ways that there are two electrons in the valence energy level. Strontium also has electrons occupying v free energy levels.
- Sr: xisouthward two2s 2twop 6threedue south 23p 6fours 2iiid ten4p vi5s 2
You lot can imagine that with the increase in the number of free energy levels, the size of the atom must increment. The increase in the number of energy levels in the electron cloud takes upwardly more space.
Therefore, the trend inside a group or family unit on the periodic table is that the atomic size increases with increased number of energy levels. The Periodic Tabular array below shows the trend of atomic size for groups. The arrow indicates the management of the increase.
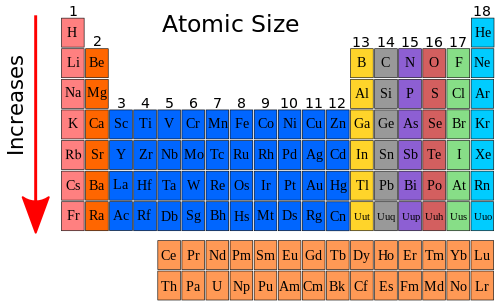
Diminutive Size in a Flow Decreases from Left to Correct [edit | edit source]
In social club to determine the trend for the periods, nosotros need to expect at the number of protons (nuclear charge), the number of energy levels, and the shielding consequence. For a row in the Periodic Table, the atomic number still increases (as it did for the groups) and thus the number of protons would increase. When we examine the free energy levels for Period 2, we find that the outermost free energy level does not alter as we increase the number of electrons. In Period 2, each additional electron goes into the second energy level. So the number of energy levels does not go up. Equally we move from left to right beyond a period, the number of electrons in the outer energy level increases only it is the same outer energy level. Table 10.3 shows the electron configuration for the elements in Catamenia two.
| Element | # of protons | Electron Configuration |
|---|---|---|
| Lithium (Li) | three | anesouthward ii2s 1 |
| Beryllium (Be) | iv | 1s 2twos 2 |
| Boron (B) | five | onedue south 22due south 2iip 1 |
| Carbon (C) | 6 | 1s ii2due south 22p 2 |
| Nitrogen (N) | seven | ones ii2s 2twop 3 |
| Oxygen (O) | eight | 1southward 22s ii2p 4 |
| Fluorine (F) | 9 | isouth 22s 22p 5 |
Looking at the elements in Menses 2, the number of protons increases from lithium with three protons, to fluorine with nine protons. Therefore, the nuclear charge increases beyond a menses. Meanwhile, the number of energy levels occupied by electrons remains the aforementioned. The numbers of electrons in the outermost energy level increases from left to right along a period just how will this touch on the radius? We know that every 1 of the elements in row #2 has two electrons in their inner energy level (2 core electrons). The core electrons shield the outer electrons from the charge of the nucleus. With lithium, there are two cadre electrons and one valence electron and so those two cadre electrons volition shield the one outer electron. In beryllium, there are four protons existence shielded by the 1due south 2 electrons. With the increasing number of protons attracting the outer electrons and the same shielding from the core electrons, the valence electrons are pulled closer to the nucleus, making the atom smaller.
In group 17, the first chemical element in the group is fluorine. With fluorine, there are ix protons and ix electrons. The electronic configuration is 1s 2twosouthward iiiip 5. However, there are still the same core electrons as with lithium and beryllium, that is, the 1south ii electrons. Since at that place are more protons, there is an increase in the nuclear charge. With an increase in nuclear charge, there is an increment in the pull betwixt the protons and the outer level, pulling the outer electrons toward the nucleus. The amount of shielding from the nucleus does non increase because the number of cadre electrons remains the aforementioned (1due south 2 for this period). The net outcome is that the diminutive size decreases going across the row. In Table ten.4, the values are shown for the atomic radii for the row starting at lithium and catastrophe with fluorine plus a visual representation to capeesh the size change in a group in the Periodic Tabular array.
| Element | Atomic Radii | Visual |
|---|---|---|
| Li | 123 pm |  |
| Be | 111 pm |  |
| B | 86 pm |  |
| C | 77 pm |  |
| Northward | 74 pm |  |
| O | 73 pm |  |
| F | 72 pm |  |
Let'southward add this new tendency to the Periodic Table. Expect at the diagram below of our new "periodic tendency table". In the diagram y'all will notice that the trend arrow for the period shows the atomic radii increase going from right to left, which is the same as decreasing from left to correct.

Considering all the data well-nigh atomic size, you lot will recognize that the largest atom on the periodic table is all the way to the left and all the way to the bottom, francium, #87, and the smallest atom is all the way to the right and all the way to the pinnacle, helium, #2.
The fact that the atoms become larger equally you motion downward in a family unit is probably exactly what y'all expected before you fifty-fifty read this section, but the fact that the atoms get smaller as you move to the right across a flow is nigh likely a large surprise. Make sure y'all understand this trend and the reasons for it.
For the Transition Elements, the Trend is Less Systematic [edit | edit source]
A full general trend for atomic radii in the Periodic Table would look like to that found in the diagram beneath. The elements with the smallest atomic radii are to the upper right; those with the largest atomic radii are to the lower left.
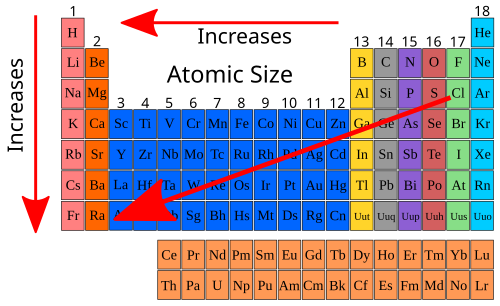
Until now, we have worked solely with the primary group metals. Let's look at our three factors and see how these factors fit the transition metal serial. Looking at the first row of the transition metals, the 3d row, Table ten.v shows the number of protons in each of the x elements in this row. The number of protons is increasing so the nuclear charge is increasing.
| Element | # of protons | Electron Configuration |
|---|---|---|
| Scandium (Sc) | 21 | [Ar]iiid 14southward 2 |
| Titanium (Ti) | 22 | [Ar]3d 24s 2 |
| Vanadium (V) | 23 | [Ar]3d 34southward two |
| Chromium (Cr) | 24 | [Ar]3d five4s ane |
| Manganese (Mn) | 25 | [Ar]3d five4s 2 |
| Fe (Fe) | 26 | [Ar]iiid 6fours 2 |
| Cobalt (Co) | 27 | [Ar]3d seven4s 2 |
| Nickel (Ni) | 28 | [Ar]3d 84south 2 |
| Copper (Cu) | 29 | [Ar]3d 104s 1 |
| Zinc (Zn) | 30 | [Ar]iiid 10ivsouthward two |
The number of electrons are increasing, but in a particular fashion. Nosotros know that as the number of electrons increases going across a period, there is more than pull of these electrons toward the nucleus. However, with the d−electrons, there is some added electron-electron repulsion. Take a look at Tabular array 10.5 and notation the unusual electron configuration of chromium.
In chromium, in that location is a promotion of ane of the foursouthward electrons to half fill the 3d sublevel, the electron-electron repulsions are less and the atomic size is smaller. The opposite holds true for the latter part of the row. Table 10.six shows the get-go row of the transition metals along with their size.
| Element | Atomic Radii |
|---|---|
| Scandium (Sc) | 164 pm |
| Titanium (Ti) | 147 pm |
| Vanadium (Five) | 135 pm |
| Chromium (Cr) | 129 pm |
| Manganese (Mn) | 137 pm |
| Iron (Fe) | 126 pm |
| Cobalt (Co) | 125 pm |
| Nickel (Ni) | 125 pm |
| Copper (Cu) | 128 pm |
| Zinc (Zn) | 137 pm |
We tin see the trend in the threed transition metals isn't quite equally systematic as with the main grouping elements.
Lesson Summary [edit | edit source]
- Diminutive size is the distance from the nucleus to the valence shell where the valence electrons are located.
- Atomic size is difficult to measure out considering it has no definite purlieus. The electrons surrounding the nucleus exist in an electron deject. You tin can predict the probability of where the electrons are merely non their verbal location.
- Atomic size is adamant indirectly.
- Diminutive radius is a more definite and measureable manner of defining atomic size. Information technology is the distance from the center of one cantlet to the center of another atom in a homonuclear diatomic molecule.
- Rutherford led the manner to determining the size of the cantlet with his gold foil experiment.
- There are iii factors that aid in the prediction of the trends in the Periodic Table: number of protons in the nucleus, number of shells, and shielding result.
- The atomic size increases from the peak to the bottom in whatever grouping every bit a result of increases in all of the 3 factors. (Equally the number of free energy levels increases, the size must increase.)
- Going across a period (from left to right), the number of protons increases and therefore the nuclear accuse increases. (Going across a period, the number of electron free energy levels remains the same merely the number of electrons increases inside these energy levels. Therefore the electrons are pulled in closer to the nucleus.)
- Shielding is relatively constant since the core electrons remain the same.
- The tendency in the periodic table is that as y'all move across the Periodic Table from left to right, the atomic radii decrease. This trend is not every bit systematic for the transition metals because other factors come into play.
Review Questions [edit | edit source]
- Why is the atomic size considered to take "no definite boundary"?
- How is atomic size measured?
- (a) using a spectrophotomer
- (b) using a tiny ruler (called a nano ruler)
- (c) indirectly
- (d) directly
- Draw a visual representation of the atomic radii of an iodine molecule.
- Which of the following would be smaller?
- (a) In or Ga
- (b) Grand or Cs
- (c) Te or Po
- Explicate in your own words why Iodine is larger than Bromine.
- What three factors determine the trend of atomic size going down a group?
- What groups tend to prove this trend?
- Which of the following would have the largest diminutive radii?
- (a) Si
- (b) C
- (c) Sn
- (d) Atomic number 82
- Which of the following would have the smallest atomic radius?
- (a) iisouthward ii
- (b) 4s 24p 3
- (c) 2s ii2p 4
- (d) fours 2
- Arrange the following in order of increasing atomic radii: Tl, B, Ga, Al, In.
- Conform the following in order of increasing atomic radii: Ge, Sn, C.
- Which of the post-obit would be larger?
- (a) Rb or Sn
- (b) Ca or Every bit
- Place the following in order of increasing atomic radii: Mg, Cl, South, Na.
- Depict the atomic size tendency for the rows in the Periodic Table.
- Depict a visual representation of the periodic table describing the trend of diminutive size.
- Which of the following would accept the largest atomic radii?
- (a) Sr
- (b) Sn
- (c) Rb
- (d) In
- Which of the following would take the smallest diminutive radii?
- (a) Yard
- (b) Kr
- (c) Ga
- (d) Ge
- Place the following elements in gild of increasing atomic radii: In, Ca, Mg, Sb, Xe.
- Place the following elements in order of decreasing atomic radii: Al, Ge, Sr, Bi, Cs.
- Knowing the trend for the rows, what would you predict to be the effect on the atomic radius if an atom were to gain an electron? Use an example in your explanation.
- Knowing the trend for the rows, what would you predict to be the event on the atomic radius if the atom were to lose an electron? Use an example in your caption.
Vocabulary [edit | edit source]
- atomic radius
- Ane-half the distance between the centers of the 2 atoms of a homonuclear molecule.
- diminutive size
- Diminutive size is the distance from the nucleus to the valence shell where the valence electrons are located.
- electron-electron repulsion
- The separation that occurs because electrons have the same accuse.
- nuclear charge
- The number of protons in the nucleus.
- shielding outcome
- The core electrons in an cantlet interfere with the attraction of the nucleus for the outermost electrons.
This material was adapted from the original CK-12 book that can be constitute here. This work is licensed nether the Creative Commons Attribution-Share Akin iii.0 Us License
How Do You Think The Size Of Atoms Will Change From Top To Bottom Within A Chemical Family?,
Source: https://en.wikibooks.org/wiki/High_School_Chemistry/Atomic_Size
Posted by: chancesteranded.blogspot.com


0 Response to "How Do You Think The Size Of Atoms Will Change From Top To Bottom Within A Chemical Family?"
Post a Comment